Information for referring physicians about the Angelman Syndrome CSF Study (AS CSF Study)
Information below is provided by Biogen
Background
At present very few biomarkers for AS have been identified. This study includes collection of CSF and blood samples from individuals diagnosed with AS or dup15q syndrome in efforts to find potential biomarkers to help us learn more about AS and information for potential development of investigational drugs for future AS clinical trials. This study does not involve receiving any investigational drug.
We are reaching out to you as a healthcare professional who actively treats patients with AS and/or dup15q syndrome who may be able to help us identify and refer eligible patients for participation in this important study. A key eligibility factor is patients must be scheduled for a procedure unrelated to the study that involves the use of general anesthesia or conscious sedation, which is necessary for the CSF collection for this study.
Study Design and Assessments
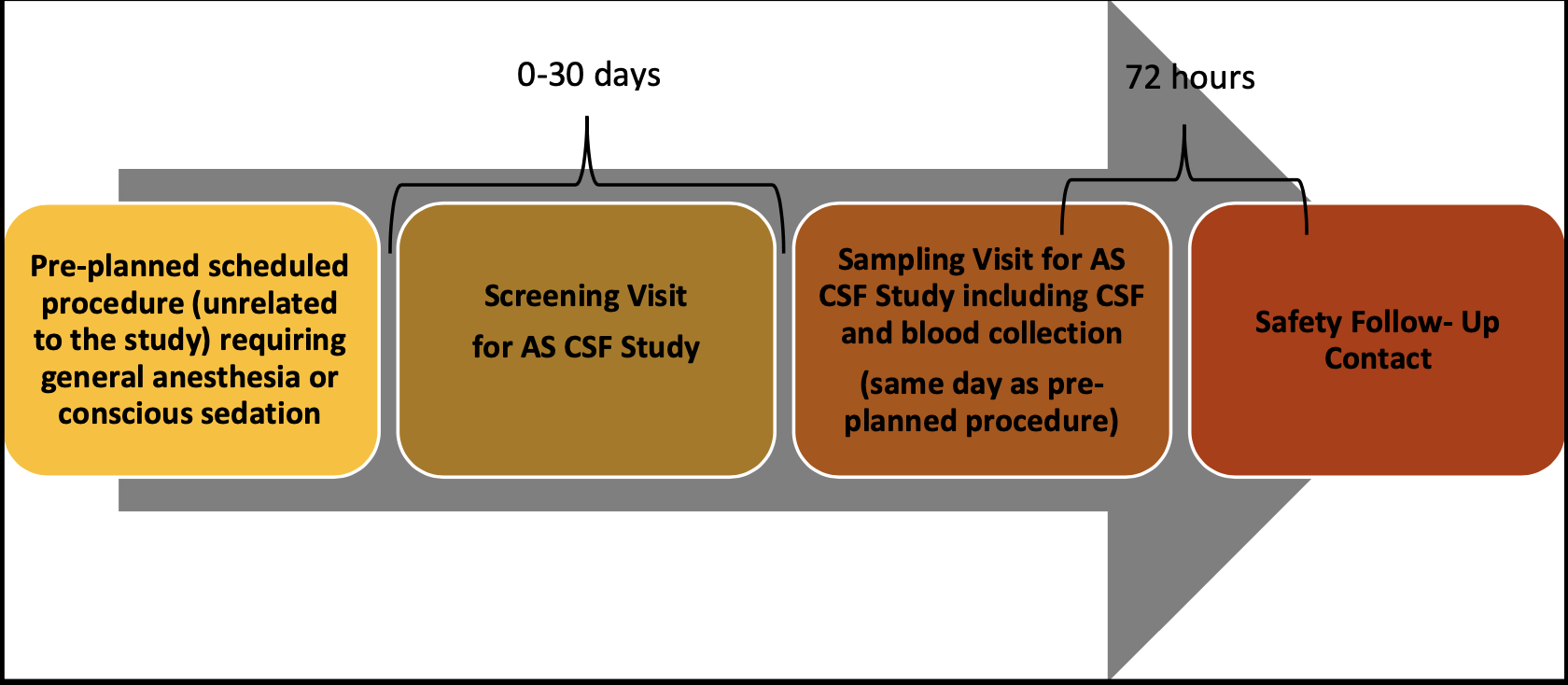
Monitoring
Participants will need to visit the study clinic at least 2 times for study assessments. Study visits include the screening visit, sampling visit and a 72-hour follow-up contact (either by phone or in person). During the study, the trained study team professionals will conduct the study assessments including collecting CSF via a lumbar puncture in addition to blood samples from the study participant while they are sedated for an already planned medical procedure.
Eligibility
To take part, candidates must meet the following key criteria (other criteria may also apply):
- Genetically confirmed diagnosis of AS or dup15q syndrome
- Ages up to 50 years old inclusive
- Are planning to have a procedure that will involve the use of general anesthesia or conscious sedation
- Legally Authorized Representative is willing to comply with the study requirements
All study-related assessments will be provided at no cost to study participants. Study participants may be eligible to receive a stipend for certain visits and reimbursement for some travel expenses. Participation is voluntary and participants can choose to leave the study at any point without penalty.
Refer a Patient
We ask you to please contact the study team if you are treating AS or dup15q who you think may be eligible. You may also contact the study team at any time for further information.
AS CSF Collection Study Sites
Rush University Medical Center
Haley Richter at Haley_E_Richter@Rush.edu / (312) 942-9645
Vanderbilt University Medical Center
Judy Jenkins RN, MSN, CCRP at (615) 936-0171
Rady Children’s Hospital San Diego
Alan Guevara at AGuevara1@rchsd.org / (858) 966-1700 EXT 1543
Boston Children’s Hospital
Kimberly Parkin at kimberly.parkin@childrens.harvard.edu / (617) 919-6897